Abstract
Background New therapies, including immunomodulatory drugs (IMiD), proteasome inhibitors (PI), and anti CD38 monoclonal antibodies (aCD38) have changed the outcomes of multiple myeloma (MM) patients. However, MM remains incurable and patients relapse and become refractory to most therapies. As aCD38 are increasingly used in early lines of therapies, retreatment of patients with aCD38-based combinations in later lines is an area of focus. Data are currently limited to address this need (1,2). The EMMY study, a large cohort designed to assess the epidemiology and real-life management of MM, can explore the real-life use and efficacy of aCD38-based retreatment.
Methods EMMY is a non-interventional, prospective dynamic cohort study conducted in 72 IFM (Intergroupe Francophone du Myélome, sponsor) centres in France since 2017. Each year, any patient initiating treatment (ttt) for MM over a 3-month observation period, from October to December, is included (800 to 1000 additional patients (pts) each year; 3616 pts included at the end of 2020) and data are updated annually from hospital records up to 2021.
Pts who initiated a second line of ttt with aCD38-based combinations after a first exposure to daratumumab (DARA) or isatuximab (ISA) were identified and described. Progression-free survival (PFS) and overall survival (OS) were described for the overall population and by line of ttt, combination of aCD38 (IMID, PI, Others), consecutive or non-consecutive aCD38 lines and aCD38 refractory status.
Results Among EMMY pts, 173 received 2 lines of ttt with aCD38. Median age was 69.6 years [Q1 Q3 61.6-75.2] with an ECOG 0-1 for 75.2% pts, a high cytogenetic risk for 37.5% (n=30/80), an ISS of 1/2/3 for 20.2%, 22.3% and 56.4%, respectively and at least one comorbidity for 36.4%.
Retreatment with aCD38 consisted in DARA after DARA for 95 pts (55%), DARA-ISA for 71 pts (41%) and ISA-DARA for 7 pts (4%). Lines of aCD38 were consecutive for 79 pts (45.7%) and non-consecutive for the others (1 middle line in 45 pts (26%) and 2+ in 49 pts (28.3%)). Median duration between consecutives lines was 0.9 month (Q1-Q3 0.1-5.9) and 15.5 months (Q1-Q3 9.2-24.4) in pts with non-consecutive lines. Pts had previously received 1 line of ttt (n=7, 4%), 2 (n=23, 13.3%), 3 (n=30, 17.3%), 4 (n=39, 22.5%), 5 (n=28, 16.2%) and 6+ (n=46, 26.6%), respectively. Most had previously received bortezomib (n=171, 99%), carfilzomib [carfi] (n=99, 58%), lenalidomide [len] (93%, n=155), pomalidomide (pom) (104, 62%), thalidomide (n=78, 47%), cyclophosphamide (n=85, 53%) and 108 had a prior autologous stem cell transplantation (62%). Of them, 127 patients (73%) were considered aCD38 refractory.
When reused, aCD38 was combined to IMID in 119 pts (68.8%) including lena-dex (n=35, 20.3%) and pom-dex (n=84, 48.6%), to IP in 37 pts (21.4%) (carfi: 100%) or to other drugs in 17 pts (9.8%), including monotherapy in 5 pts.
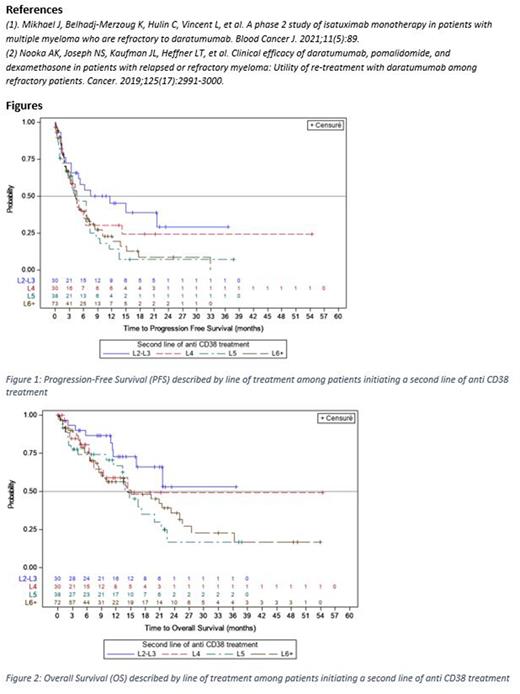
For all aCD38-retreated pts, mPFS was estimated at 4.7 months (m) (95 CI% 3.8-6.5); 11.6 m (3.4 to not reached) in L2/L3 pts, 4.6m (2.3-6.5), 4.9m (2.7-6.7) and 4.3m (3.2-6.6) in the L4, L5 and L6+ pts, respectively (Figure 1). mPFS was 4.5m (3.5-6.7) in the aCD38-pom-dex pts, 3.8m (1.8-7.2) in the aCD38-lena-dex pts, 6.5m (4.6-8.7) in the aCD38-PI pts and 2.3m (0.9-6.5) in the aCD38-other pts. mPFS was 6.5m (4.0-7.5) in pts with aCD38 consecutive lines, 4.6m (2.6-6.0) and 3.9m (2.8-6.6) in pts with non-consecutive 1 and 2+ middle lines, respectively. mPFS was 7.2m (3.4 to not reached) in aCD38 non refractory pts and 4.6m (3.7-6.0) in pts refractory to aCD38.
mOS was estimated at 16.5m (13.9-21.6) for all pts; mOS not reached for L2/L3 pts, and was 14.4m, 13.9m and 15.1m in L4, L5, L6+ pts, respectively (Figure 2). mOS was 21.3m in pts with aCD38 consecutive line, 16.2m and 9.1m in pts with non-consecutive 1 and 2+ middle lines. mOS was 14.2m in aCD38-pom-dex pts, 25.1m in aCD38-lena-dex pts, 21.5m in aCD38-PI pts and 16.5m in aCD38-other pts.
Conclusion These results are among the first assessment of aCD38 re-use of in a large sample of real-life patients (n=173). Patients were extensively treated when they received a second line of aCD38, most of them were refractory to aCD38 and had received 4+ previous lines for MM. The most meaningful results are found in the early lines of treatment (L2/L3) in patients not refractory to aCD38. These findings need to be further investigated while the use of aCD38 is emerging as a first-line treatment for newly diagnosed patients.
Disclosures
Hulin:Amgen: Honoraria; Takeda: Honoraria; BMS: Honoraria; GSK: Honoraria; Sanofi: Honoraria; Janssen: Honoraria. Perrot:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria. Macro:Janssen: Honoraria, Other: Travel/accommodation, Research Funding; Sanofi: Honoraria; GSK: Honoraria; Takeda: Honoraria, Other: Travel/accommodation, Research Funding. Leleu:Amgen, Merck, BMS, GSK, Janssen, Oncopeptide, Takeda, Roche, Novartis, AbbVie, Sanofi, Gilead, Pfizer, Harpoon Therapeutic, Regeneron, Iteos: Consultancy, Honoraria; Pfizer: Honoraria; Amgen: Honoraria; Sanofi: Honoraria; Takeda: Honoraria; BMS: Honoraria; Janssen: Honoraria; Amgen, BMS/Celgene, Janssen, Takeda, Novartis, Sanofi, Merck, Oncopeptide, Karyopharm, Roche, Abbvie, Carsgen, GSK, and Harpoon Therapeutics: Honoraria. Karlin:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial Support travel & scientific meetings; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial Support travel & scientific meetings; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Manier:AbbVie: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnology: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Regeneron: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Vincent:BMS: Membership on an entity's Board of Directors or advisory committees, Other: Financing meeting participation; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financing meeting participation; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Financing meeting participation; Sanofi: Honoraria, Other: Financing meeting participation; Pfizer: Other: Financing meeting participation, congress participation; Sandoz: Other: Education course paid by sandoz; Amgen: Membership on an entity's Board of Directors or advisory committees. Texier:Kappa Sante: Current Employment. Decaux:BMS: Honoraria; GSK: Honoraria; Sanofi: Honoraria; Takeda: Honoraria; Roche: Honoraria; Gilead: Honoraria; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal